Nanotechnology Research
Tetrachloroethylene (TCE) is a common contaminate in groundwater that can cause anything from mild skin irritations to cancer. Currently, methods for removing this highly carcinogenic containment and others like it are inefficient and ineffective. Granular activated carbon (GAC) is often used to remove these contaminants by adsorbing them onto its surface. This simple method does not truly solve the contamination issue as it only relocates the carcinogen to another location. This could be especially problematic because TCE and many other harmful contaminates are classified as volatile organic compounds (VOCs) and therefore, vaporize readily when exposed to the air, creating an even more harmful situation than with which to begin (Chaplin 2012).
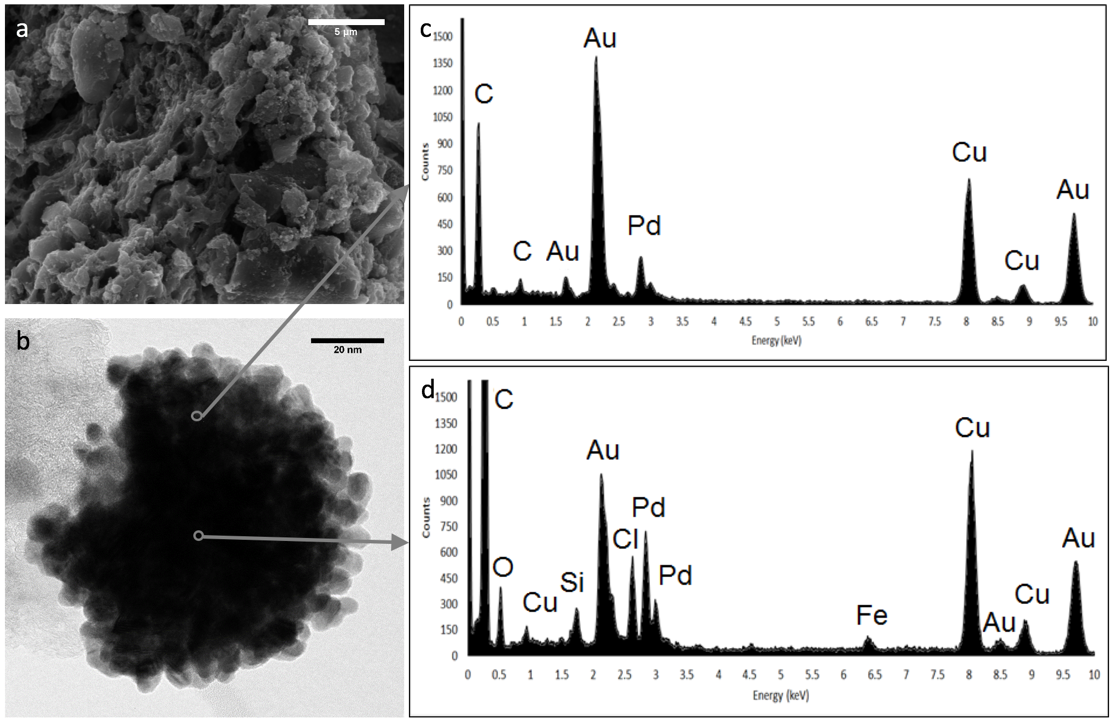
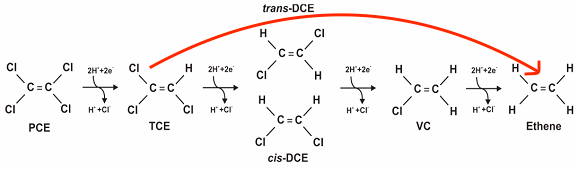
My research focuses on studying and synthesizing a novel palladium and gold catalyst that is supported by carbon, either graphene or GAC (Fig1.) to degrade TCE and other VOCs into ethene and ethane while "skipping" over the more harmful products that lie inbetween, like vinyl chloride (VC). This process is called hydrodechlorination (Fig2.).
I have specifically studied the optical properties of our catalyst and what parts of synthesis effect our nanoparticles (NPs). To synthesize our catalyst, gold and palladium are sonicated with acetone and left to react for 24 hours at a certain temperature. Iíve looked at how the time of sonication and time left for our solution to react effects the synthesis of nanoparticles. To determine if, how many, or what size our NPs are, I use UV-spectroscopy (Img1.) to study the wavelength of light absorbed by our product.

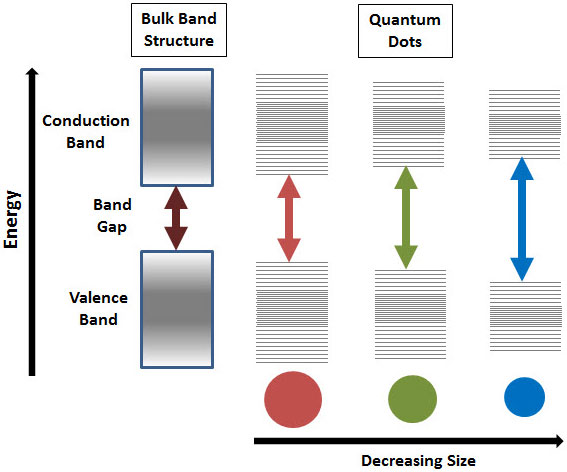
NPs undergo quantum confinement which leads to interesting changes in optical and electronic properties due to an increase of bandgap as the size of the nanoparticle decreases. Depending on the change in optical properties of the nanoparticles, the size or existence of NPs can be determined.
Studying the properties of these nanoparticles is important in understanding how our catalyst works and to assure that is not further contaminating the environment or water. Our gold and palladium, carbon supported nanocatalysts are a promising solution for industry and environmental clean up that not only remove toxic contaminates like TCE, but degrade them into something else entirely.
